Some N₂H4 is placed in a 1.0 L container, which is sealed, and raised to 1273 K. After the reaction, chemical analysis shows that the concentration of H₂ is 0.20 M , N₂is 0.10 M , and N₂H4 is 0.10 M . Calculate K c for:
N₂H4 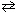 N₂+ 2 H₂
N₂+ 2 H₂
Definitions:
Assembling Cost
The expenses incurred in the process of putting together components or parts to manufacture a finished product.
Units-Of-Production Method
A depreciation method in which an asset's cost is allocated based on the number of units it produces.
Salvage Value
Salvage value is the estimated residual value of an asset at the end of its useful life.
Units-Of-Production Method
A depreciation method that allocates the cost of an asset over its useful life based on the number of units it produces.
Q9: In the titration of 50.0 mL of
Q10: Which of the following titration curves listed
Q12: A 350 mL volume of 0.150 M
Q25: Refer to Exhibit 13-12. What is the
Q31: How many unit cells share an atom
Q46: What is the conjugate acid of the
Q48: Consider the changes in acid strengths in
Q90: Refer to Exhibit 11-4. How many aluminum
Q127: Refer to Exhibit 13-4. What is the
Q179: The pH of a 0.050 M solution