At a certain temperature, for the reaction NH ₄Cl (s) 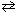 NH₃(g) + HCl (g) , K p = 9.0.
NH₃(g) + HCl (g) , K p = 9.0.
What is the equilibrium partial pressure of NH₃?
Definitions:
Conflict
A situation where there is a disagreement or opposition between parties, which can stem from different views, interests, or goals.
Group Size
Refers to the number of individuals making up a group, which can impact the group's dynamics, interaction, and effectiveness.
Verbal Participation
The extent to which individuals contribute to discussions or communication through spoken words.
Conjunctive Tasks
Tasks in which group performance is limited by the performance of the poorest group member.
Q37: The gas phase reaction C<sub>2</sub>H<sub>4</sub> + Cl<sub>2</sub>→C<sub>2</sub>H<sub>4</sub>Cl<sub>2</sub>
Q54: Calculate the pH in a solution which
Q63: What is the partial pressure equilibrium constant,
Q72: At 25 ° C, 13.0 mg of
Q78: Refer to Exhibit 13-14. This reaction has
Q90: For the Haber reaction at equilibrium ,
Q96: The best measure of the spontaneity of
Q114: The reaction NO (g) + O<sub>3</sub>(g) <img
Q124: What is the energy of activation, E<sub>a</sub>,
Q135: Consider the following reaction. 3 O<sub>2</sub> (g)→2