The equilibrium constant for the reaction below is 1.7*1017 at 25 C.
H₂ (g) + Br₂(g) 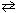 2 HBr (g) K c (25 C) = 1.7*1017
2 HBr (g) K c (25 C) = 1.7*1017
What can be stated about the relative equilibrium distribution for this mixture?
Definitions:
Bladder Distension
Bladder distension occurs when the bladder is overly filled and cannot properly empty, leading to discomfort and possible urinary complications.
Urinary Incontinence
The loss of bladder control, leading to the accidental leakage of urine.
Urinary Retention
A medical condition characterized by the inability to completely or partially empty the bladder.
Hesitancy
The delay and difficulty in initiating urination, often with a decreased strength and calibre of the urinary stream.
Q1: The second period homonuclear diatomic molecule with
Q18: Which anionic base listed below would be
Q30: What is the equilibrium constant expression for
Q73: The decomposition of hydrogen peroxide to water
Q81: Regarding acid strength, which of the following
Q131: How many sigma ( s ) bonds
Q136: In general, a process that is exothermic
Q148: For the reaction 2 O<sub>3</sub> (g)→3 O<sub>2</sub>
Q159: Arrange the following aqueous solutions in order
Q177: What is the molarity of ions in