Multiple Choice
At a certain temperature K c = 9.0 for the equilibrium N₂O4 (g) 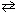 2 NO₂ . What is K c at the same temperature for:
2 NO₂ . What is K c at the same temperature for:
NO₂ (g) 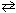 1 / 2 N₂O4 (g)
1 / 2 N₂O4 (g)
Definitions:
Related Questions
Q37: The gas phase reaction C<sub>2</sub>H<sub>4</sub> + Cl<sub>2</sub>→C<sub>2</sub>H<sub>4</sub>Cl<sub>2</sub>
Q37: Exhibit 15-2 The pH of a solution
Q40: If the reaction A + B→C has
Q40: How many grams of NaCl are required
Q49: What is the pH of 2.0 liters
Q54: For which of the following reactions is
Q73: The combustion of a 3.06 g sample
Q137: At equilibrium, the system N₂O<sub>4</sub> (g) <img
Q171: Refer to Exhibit 14-7. What is the
Q208: At 457 K, the value of K