What is the value of the concentration equilibrium constant, K c (@1000 K) , for the following reaction?
CO (g) + Cl₂ (g) 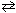 COCl₂ (g) K p = 3.9*10- 2 (@ 1000 K)
COCl₂ (g) K p = 3.9*10- 2 (@ 1000 K)
Definitions:
Depository Institution
A financial institution that accepts deposits from individuals and provides loan services.
Investment Bank
A financial institution that provides advisory services, financing, and investment services to individuals, corporations, and governments.
Short-term Debt Securities
Financial instruments that represent borrowed funds which must be repaid within a short time frame, typically less than one year.
Money Markets
Financial markets for short-term borrowing and lending, dealing with assets that have high liquidity and short maturities.
Q35: What is the Molarity of a 5.00%
Q56: Which species listed below is the conjugate
Q58: Which of the following molecules can form
Q60: What is the pH of an aqueous
Q76: What is the vapor pressure of a
Q86: The density of a solution that is
Q89: The basic repeating three-dimensional pattern of a
Q104: What is the Pb<sup>2+</sup> concentration in water
Q117: For the reaction Fe (s) + 4
Q120: What is the H<sub>3</sub>O<sup>+</sup> ion concentration in