At 1000 K, the value of the K eq for the following reaction equals 0.338.
2 SO3(g) 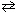 2 SO₂ (g) + O₂(g) K c = 0.338
2 SO₂ (g) + O₂(g) K c = 0.338
If the partial pressure values for these three components at a single moment were:
P SO3 = 0.16 atm P SO2 = 0.41 atm PO2 = 2.5 atm What can be stated about the reaction quotient , Q , and the direction that the reaction is preceding?
Definitions:
Muscular Tubes
Structures composed of smooth muscle, designed to transport substances through contractions, found in organs like the intestines and blood vessels.
Hernia
A condition in which part of an organ is displaced and protrudes through the wall of the cavity containing it, often creating a bulge.
Renal Artery
A blood vessel that carries oxygenated blood to the kidneys.
Bacteriologic Culture
The laboratory process of growing bacteria from a sample to identify a possible infection.
Q3: What is the pH of a solution
Q18: The solubility of Tl<sub>2</sub>CrO<sub>4</sub> in pure water
Q49: The y-intercept on a second order Time-Concentration
Q58: What is the molality of a solution
Q66: A crystal diffracts x-rays (£ = 154
Q67: Aluminum metal crystallizes in a face centered
Q119: Refer to Exhibit 13-10. What is the
Q119: Two anions act as bases. Anion A<sup>
Q124: For the reaction 2 H₂ O (g)
Q167: The solubility of a gas in water