Consider the following chemical reaction at equilibrium:
2 CH4 (g) + O₂(g) 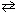 2 CO (g) + 4 H₂ (g) What is the effect of adding more O₂to this system at equilibrium?
2 CO (g) + 4 H₂ (g) What is the effect of adding more O₂to this system at equilibrium?
Definitions:
Guilt-proneness
The predisposition of an individual to feel guilt for wrongdoing, reflecting a sensitivity to the ethical or moral implications of their actions.
Higher
Typically used to denote something that is above average or superior in quality, position, or importance.
Rated
Assigned a classification or standard often based on quality, performance, or suitability.
Narcissism
Narcissism is a personality trait characterized by an excessive preoccupation with oneself, a need for admiration, and a lack of empathy for others.
Q17: Calculate the pH of a solution after
Q33: Three molecules are listed below. Will hydrogen
Q44: A 25.0 mL volume of 0.100 M
Q50: What is the pH after the addition
Q76: What is the pH of aqueous 0.10
Q80: What intermolecular force(s) is(are) present among molecules
Q87: Calcium fluoride, CaF<sub>2</sub>, has a unit cell
Q121: A sample of 20.0 g of a
Q142: Refer to Exhibit 14-5. What would be
Q142: At 1000 ° C, cyclobutane (C<sub>4</sub>H<sub>8</sub>) decomposes