For the reaction 2 NH₃(g) 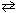 N₂(g) + 3 H₂ (g) , what would you expect if 0.6 atm of inert neon gas is added at constant volume to the equilibrium mixture of gases?
N₂(g) + 3 H₂ (g) , what would you expect if 0.6 atm of inert neon gas is added at constant volume to the equilibrium mixture of gases?
Definitions:
Imbalance Of Power
A situation where there is a disproportionate distribution of power or resources among individuals or groups, often leading to unequal treatment or outcomes.
Policing Mission
Refers to the fundamental objectives and goals of policing organizations, which typically include maintaining public order, enforcing laws, and preventing, detecting, and investigating criminal activities.
Necessary
Refers to something that is needed or required.
Typographies
The art or process of setting and arranging types and printing from them, or the style and appearance of printed matter.
Q9: At 20 ° C the Henry's law
Q12: Which of the following chemical reactions has
Q19: Iodine-123 is used to study thyroid gland
Q34: K <sub>a</sub> for HF is 3.5×10<sup> -
Q59: Refer to Exhibit 15-5. What is the
Q60: What is the pH of an aqueous
Q85: The mole fraction of acetone in a
Q95: For most substances, the enthalpy of the
Q97: Which one of the following systems shows
Q170: How many grams of NH<sub>3</sub> are present