At 600 C, K p = 0.57 for the endothermic reaction:
CO₂ (g) + H₂ (g) 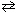 CO (g) + H₂ O (g) The value of K p at 300 C is:
CO (g) + H₂ O (g) The value of K p at 300 C is:
Definitions:
Marginal Products
The additional output that results from the use of an additional unit of a productive input, holding other inputs constant.
Pooling Equilibrium
A situation in a signaling game where different types cannot be distinguished by the actions they take, leading everyone to pool together and behave identically.
Low-productivity Workers
Employees who generate less output per unit of input compared to others, often leading to less efficiency within an organization.
Moral Hazard
The situation where one party is more likely to take risks because another party bears the cost of those risks.
Q10: A reaction in solution, A + B→P,
Q14: In the titration of 0.100 M HCl
Q34: What is the vapor pressure lowering ,
Q53: Benzoic acid is weak with K <sub>a</sub>
Q65: Tritium is radioactive and decays by a
Q69: Regarding acid strength, which of the following
Q80: Which thermodynamic statement listed below would confirm
Q81: How many grams of Na<sub>2</sub>SO<sub>4</sub> are required
Q106: The decomposition of N<sub>2</sub>O<sub>5</sub> proceeds according to
Q118: How many moles of sucrose, C<sub>12</sub>H<sub>22</sub>O<sub>11</sub>, is