For the high temperature equilibrium system, 2 H₂ O (g) 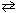 2 H₂ (g) + O₂(g) , what will be the effect on the equilibrium concentration of O₂(g) upon:
2 H₂ (g) + O₂(g) , what will be the effect on the equilibrium concentration of O₂(g) upon:
(1) adding H₂ O (g) and (2) decreasing the pressure of the system?
Definitions:
Service Revenue
Income earned by a company through the provision of services to customers or clients.
Notes Payable
A written promise to pay a specified amount of money, along with interest, by a certain date.
Interest Expense
Expenses that a company faces for using borrowed capital, usually shown on the profit and loss statement.
Owner's Equity
The residual interest in the assets of an entity that remains after deducting its liabilities. It represents the ownership interest of the shareholders in a company.
Q29: The decomposition of hydrogen iodide is shown
Q38: Arrange the following elements in order of
Q39: What is the equilibrium constant expression for
Q51: From the D E given for 25
Q69: How many grams of Na<sub>2</sub>CO<sub>3</sub> are required
Q85: The mole fraction of acetone in a
Q94: K p for COCl₂ (g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8714/.jpg"
Q108: Which of the substances listed below is
Q110: A 3.38 molar solution of cesium chloride
Q182: In water, the solubility of Al(OH)<sub>3</sub> is