Consider the following endothermic reaction at equilibrium:
2 N₂(g) + 6 H₂ O 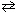 4 NH₃(g) + 3 O₂(g) Which statement is true?
4 NH₃(g) + 3 O₂(g) Which statement is true?
Definitions:
Diabetes
A metabolic disease characterized by high blood sugar levels over a prolonged period, which can result in serious health complications.
Famine
An extreme scarcity of food, affecting a large number of people over a wide area, leading to malnutrition, starvation, and death.
Tissue
A group or layer of cells that together perform specific functions and form the material substances of animals and plants.
Organs
Structures composed of multiple types of tissues that work together to perform specific functions within an organism's body.
Q14: A 3.60 m solution of KBr (molar
Q42: Given:<br>2 Cl₂ (g) + 2 H₂ O
Q48: A metal that has a radius of
Q52: Mg(OH)<sub>2</sub> ( K <sub>sp</sub> = 8.9×10<sup> -
Q56: Refer to Exhibit 13-4. What units are
Q94: Identify the Lewis acid in the following
Q101: If the molar solubility of Silver Phosphate,
Q107: What is the definition for molarity ?<br>A)
Q132: What is the value of K p
Q137: Acetic acid has K <sub>a</sub> = 1.8×10<sup>