Which of the items listed below would increase the yield of NO₂ in the following equilibrium reaction?
2 NO (g) + O₂(g) 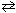 2 NO₂ (g) (exothermic)
2 NO₂ (g) (exothermic)
I. Add more O₂to the equilibrium mixture.
II. Warm the reaction mixture.
III. Increase the pressure by decreasing the volume of the reaction vessel.
Definitions:
Duty of Notification
An agent’s obligation to inform the principal of the agent’s actions on the principal’s behalf and of all relevant information.
Communicate Offers
The process through which an offeror makes their proposal known to the offeree, initiating the possibility of forming a contract.
Indemnification
Indemnification is the process by which one party agrees to compensate another for any losses or damages suffered, often as part of a contractual agreement.
Breach of Contract
The failure to perform any term of a contract, written or oral, without a legitimate legal excuse.
Q30: If only work of expansion is done,
Q31: The term that describes the situation where
Q73: The decomposition of hydrogen peroxide to water
Q77: What is the Calcium hydroxide concentration, [Ca(OH)<sub>2</sub>],
Q83: For the reaction, PCl<sub>5</sub> (g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8714/.jpg"
Q103: The reaction X + 2 Y A
Q112: How many grams of solute are present
Q141: For NO + NO<sub>2</sub> + O<sub>2</sub>→NO<sub>3</sub> +
Q146: The rate of reaction is influenced by:<br>A)
Q161: A dilute solution of HBr is found