What is the equilibrium constant, K p , for the reaction below if the equilibrium partial pressures are P NO = 0.0950 atm, P Cl2 = 0.171 atm, and P NOCl = 0.280 atm?
2 NO (g) + Cl₂ (g) 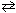 2 NOCl (g)
2 NOCl (g)
Definitions:
Avoidant Attachment
A type of attachment style where individuals maintain their distance from others and avoid close emotional bonds.
Infantile Amnesia
The inability of adults to recall memories from early childhood, typically before the age of 3-4 years.
Egocentrism
Lacking the ability to separate one's own point of view from another individual's perspective.
Attachment
An emotional bond between individuals, particularly seen in relationships between parents and children, affecting long-term development and emotional wellbeing.
Q1: At 100 C, the equilibrium constant for
Q3: A biochemist studying the breakdown in soil
Q31: Calculate the pOH of a solution made
Q34: What is the volume of 0.0175 M
Q37: For the reaction I<sub>2</sub> (g) + Cl₂
Q53: Benzoic acid is weak with K <sub>a</sub>
Q61: Which ionic compound(s) listed below would increase
Q95: Refer to Exhibit 13-16. How long would
Q122: What is the equilibrium partial pressure of
Q192: Which system at equilibrium shifts right ?<br>I.