A reaction vessel is charged witH₂ .46 atm of NO, 2.46 atm of H₂ O and 1.23 atm of H₂ gases. Equilibrium is established as shown in the reaction below. 2 NO (g) + 2 H₂ (g) 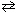 N₂(g) + 2 H₂ O (g)
N₂(g) + 2 H₂ O (g)
At equilibrium, the partial pressure of NO is 1.53 atm . What is the value of the equilibrium constant, K p ?
Definitions:
Susceptible Host
An individual or organism that is vulnerable or lacks resistance to an infectious agent, thus at risk of becoming infected.
Salmonella Poisoning
An infection caused by Salmonella bacteria, often leading to gastrointestinal illness and symptoms such as diarrhea and vomiting.
Exogenous Infection
An infection caused by pathogens introduced from the external environment into a host body, contrasting with those arising from the host's own flora.
Endogenous Infection
An infection caused by an infectious agent that is already present within the body, rather than being introduced from an external source.
Q55: What is the conjugate acid of the
Q59: At 120 ° C, the vapor pressure
Q64: Refer to Exhibit 13-9. What is the
Q65: A diamond crystal is classified as:<br>A) ionic<br>B)
Q118: How many moles of sucrose, C<sub>12</sub>H<sub>22</sub>O<sub>11</sub>, is
Q147: Which of the following molecular compounds would
Q149: Sulfur trioxide decomposes at high temperature in
Q162: An equilibrium mixture at 500 K has
Q165: A solution is prepared by mixing 4.00
Q178: A Bronsted-Lowry acid is<br>A) a proton acceptor.<br>B)