At high temperature, the following gas phase equilibrium is established:
N₂O5 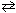 N₂O + 2 O₂Initially, 1.00 mole of N₂O5
N₂O + 2 O₂Initially, 1.00 mole of N₂O5
Assume is sealed into a 0.182 L flask. After equilibrium, the N₂O concentration is 0.50 M . Calculate K c .
Definitions:
Court Cases
Legal disputes brought for review and resolution in a court of law.
First Amendment
An amendment to the U.S. Constitution that prohibits the government from making laws that infringe upon the freedom of speech, religion, press, assembly, and petition.
Religious Freedom
The right to practice any religion of one's choice, or none, without government interference, as guaranteed by the Constitution.
Protected Expression
Forms of speech or expression that are safeguarded against government restriction or censorship under constitutional or legal standards.
Q11: What is the solubility of Pb(IO<sub>3</sub>)<sub>2</sub> in
Q31: A sample of acetic acid (approximately 0.001
Q33: Three molecules are listed below. Will hydrogen
Q40: How many grams of NaCl are required
Q48: A metal that has a radius of
Q52: Calculate K p for the reaction PCl<sub>5</sub>
Q91: At what angle are x-rays with a
Q93: The gas-phase decomposition of SO<sub>2</sub>Cl<sub>2</sub> is a
Q110: A 3.38 molar solution of cesium chloride
Q158: An amphoteric substance:<br>A) has a very low