Given that K eq = 2.4*103 for the reaction below at 2000 C and the initial partial pressure of NO equals 37.3 atm, what is the equilibrium partial pressure of NO at this temperature?
2 NO (g) 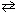 N₂(g) + O₂(g)
N₂(g) + O₂(g)
Definitions:
ASPE
A framework of accounting guidelines tailored for privately owned Canadian entities to maintain consistency in financial reporting.
IFRS
International Financial Reporting Standards, a set of accounting guidelines providing a global framework for financial statements.
Deferral Method
An accounting procedure that postpones the recognition of certain income or expenses until a later accounting period.
Financial Statements
Reports that provide an overview of a company's financial condition, including income statement, balance sheet, statement of cash flows, and statement of changes in equity.
Q30: What is the equilibrium constant expression for
Q41: Consider the following reaction and its corresponding
Q63: The reaction PCl<sub>3</sub> (g) + Cl<sub>2</sub> (g)→PCl<sub>5</sub>
Q97: Which crystal packing structure listed below provides
Q99: Consider the following reaction that follows a
Q103: What is the molality of a solution
Q106: A dilute aqueous solution of an organic
Q130: Refer to Exhibit 14-7. The solubility product
Q185: Calculate the solubility of AgI in 0.10
Q205: Consider the reaction:<br>2 HI (g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8714/.jpg"