Exhibit 14-6 Consider the reaction at equilibrium below and its corresponding equilibrium constant to answer the following question(s) . 2 NO₂ (g) 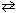 2 NO (g) + O₂(g) K p = 4.48*10- 13 A pressure of 0.45 atm of NO₂ is introduced into a container and allowed to come to equilibrium. Complete the ICE equilibrium table shown below to get started. Partial pressure S2NO₂ 2 NO O₂Initial 0.45 M Change At equilibrium
2 NO (g) + O₂(g) K p = 4.48*10- 13 A pressure of 0.45 atm of NO₂ is introduced into a container and allowed to come to equilibrium. Complete the ICE equilibrium table shown below to get started. Partial pressure S2NO₂ 2 NO O₂Initial 0.45 M Change At equilibrium
-Refer to Exhibit 14-6. Upon constructing an ICE table, what expression would be obtained that represents the equilibrium partial pressure of NO₂ as it reaches equilibrium?
Definitions:
FVTPL
Fair Value Through Profit or Loss, a classification under International Financial Reporting Standards for financial assets held for trading or designated upon initial recognition as measured at fair value, with changes in fair value recognized in profit or loss.
FVTOCI
stands for Fair Value Through Other Comprehensive Income, a classification under IFRS for financial assets that are measured at fair value with fluctuations recorded in other comprehensive income.
Share Price
The price at which a single share of a company's stock is bought or sold in the market.
ASPE
Accounting Standards for Private Enterprises, a set of accounting practices and principles for private companies in Canada.
Q11: Refer to Exhibit 16-2. What compound(s) is(are)
Q34: K <sub>a</sub> for HF is 3.5×10<sup> -
Q54: Which of the following solutions will not
Q61: What is the vapor pressure of water
Q67: Consider the following unbalanced reaction :<br>NO (g)
Q87: The oxidation number of Cl in HClO<sub>3</sub>
Q125: Which of the following salts is considered
Q125: What is the vapor pressure lowering D
Q153: The solubility of BaSO<sub>4</sub> is 1.14×10<sup> -
Q167: The solubility of a gas in water