The K c for the reaction,
PCl5 (g) 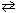 PCl3(g) + Cl₂ (g) , at a particular temperature is 4.0*10- 2 .
PCl3(g) + Cl₂ (g) , at a particular temperature is 4.0*10- 2 .
How many moles of PCl5 must be placed in an empty 1.0 liter flask to have the equilibrium concentration of Cl₂ = 0.15 M ?
Definitions:
Nudging
Nudging involves subtly guiding choices or behaviors without restricting options, often used in policy and marketing to influence decision-making.
Dictator Game
An experimental game in economics where one player, the "dictator," determines how to split a sum of money with another player.
Behavioral Economics
An area of economics that examines the influence of psychological, cognitive, emotional, cultural, and social aspects on economic decision-making.
Net Change
The difference between the closing value of a financial instrument on a given day and its closing value on the preceding day.
Q2: Benzene boils at 80.10 ° C and
Q14: A 3.60 m solution of KBr (molar
Q17: If q of the system is negative:<br>A)
Q38: Which of the following processes would be
Q49: Given the abbreviated table for selected standard
Q55: A solution of HCOOH ( K <sub>a</sub>
Q137: Concentrated red fuming Nitric acid, HNO<sub>3</sub>, is
Q146: The rate of reaction is influenced by:<br>A)
Q161: A mixture of Na<sub>2</sub>CO<sub>3</sub>, NaCl, and NiCO<sub>3</sub>
Q171: The molarity and molality of a solution:<br>A)