What would be the equilibrium constant expression for the following reaction?
Na2 CO3(s) + 2 HCl (g) 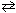 2 NaCl (aq) + CO₂ (g) + H₂ O (
2 NaCl (aq) + CO₂ (g) + H₂ O (  )
)
Definitions:
Wealth Inequality
The unequal distribution of assets among a population.
Income Quintile
A statistical measure dividing the population into five equal groups based on income levels, to analyze economic inequality.
Gini Ratio
A measure of the income distribution within a population, indicating inequality where 0 represents perfect equality and 1 represents perfect inequality.
Income Inequality
The uneven distribution of income within a population, leading to disparities in wealth and living standards.
Q2: Consider the following Bronsted-Lowry acid-base reaction:<br>C<sub>6</sub>H<sub>5</sub>NH<sub>2</sub> +
Q5: Refer to Exhibit 17-2. What is the
Q28: At 25 ° C the vapor pressure
Q46: What is the pH of the following
Q55: A solution of HCOOH ( K <sub>a</sub>
Q80: Which thermodynamic statement listed below would confirm
Q107: Which of the following iodates would be
Q117: A solution is prepared by mixing 22
Q134: The molality of an unknown aqueous solution
Q151: For the reaction COCl₂ (g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8714/.jpg"