What is the concentration of Pb 2+ ions in a solution resulting from the addition of 1.0 10 - 3 moles of Na2 CrO4 to 1.0 liter of solution containing solid PbCrO4 ( K sp = 1.8*10- 14 ) in equilibrium with its ions?
(Assume no volume change on addition of Na2 CrO4 .) PbCrO4 (s) 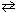 Pb 2+ (aq) + CrO₂ - (aq)
Pb 2+ (aq) + CrO₂ - (aq)
Definitions:
Owners' Equity
The residual interest in the assets of a business after deducting liabilities, essentially the ownership interest in the business.
Store Equipment
The fixtures and tools used in a retail store to display and manage products, excluding inventory.
Owners' Claims
Owners' claims refer to the interests or rights of ownership that shareholders or proprietors have in a company, including profits and assets.
Business Assets
Resources owned by a business that have economic value and can contribute to future profits, such as equipment, inventory, and patents.
Q7: The calculated value of D S is
Q7: An aqueous solution of concentrated glacial acetic
Q22: Consider the following reaction at equilibrium:<br>2 Cl₂
Q27: What is the pH of a 1.6
Q82: The only factor that causes a change
Q86: Which of the following is true?<br>A) D
Q102: What is the base ionization constant ,
Q111: In the peroxydisulfate ion, S<sub>2</sub>O<sub>8</sub><sup>2</sup> - ,
Q152: A solution is 0.100 M in AgNO<sub>3</sub>
Q179: If we compare the acid/base properties of