Exhibit 15-4 Consider dissolving NaF in water as shown in the equation below to answer the following question(s) :
NaF (s) + H₂ O ( 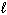 )
) 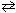 HF (aq) + NaOH (aq)
HF (aq) + NaOH (aq)
-Refer to Exhibit 15-4. What is the base ionization constant , K b , for this reaction?
K a (HF) = 6.8 10 - 4
Definitions:
Quantity Supplied
Refers to the amount of a good or service that producers are willing to sell at a given price over a specific period.
Quantity Demanded
The total amount of a good or service that consumers are willing to purchase at a given price.
Rent-Controlled Apartments
Rent-controlled apartments are rental units subject to governmental regulations that limit the amount landlords can charge for rent, often to protect tenants from rapid price increases.
Lower Rents
Lower rents refer to reduced prices for leasing properties, often as a result of market conditions, government policies, or landlord decisions.
Q26: What is the base ionization constant ,
Q27: What is the pH of a 1.6
Q52: How are the two compounds that are
Q56: For any system at equilibrium, the value
Q71: Which of the following units express(es) a
Q97: A sample of hydrochloric acid (approximately 0.10
Q105: A catalyst may be defined as:<br>A) a
Q108: An endothermic reaction CaCO<sub>3</sub>(s) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8714/.jpg" alt="An
Q148: Given:<br>H₂ O (g) + CO (g) <img
Q176: For the reaction 2 SO₂ (g) +