Consider the ionization of acetic acid as follows:CH ₃COOH + H₂ O 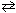 H₃O + + CH₃COO 1 - What is the effect of adding the common ion, acetate, CH₃COO - (source:
H₃O + + CH₃COO 1 - What is the effect of adding the common ion, acetate, CH₃COO - (source:
CH₃COONa) , to this equilibrium system?
Definitions:
Secure Wireless Payments
Refers to the transmission of payment information over a wireless network in a manner that is encrypted and protected from unauthorized access.
Technician
A skilled worker with specific technical knowledge, especially in the fields of science and engineering.
Trade Shows
Events where companies in a specific industry showcase and demonstrate their new products and services to potential customers and partners.
UTM
A security device that provides multiple functions, such as content filtering, antivirus, antispyware, anti-malware, firewall, and intrusion detection and prevention.
Q25: The O - O bond order in
Q43: A reaction will proceed spontaneously at any
Q44: What species are formed in the hydrolysis
Q54: For which of the following reactions is
Q62: Which molecule listed below (none are cyclic)
Q80: Which thermodynamic statement listed below would confirm
Q95: Refer to Exhibit 13-16. How long would
Q167: Given the following two reactions and their
Q173: What can be stated about the name,
Q198: Enough NO gas was placed into a