Consider the following equilibrium reaction:
NH₃+ H₂ O 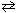 NH ₄+ + OH - K b (NH ₃) = 1.8 10 - 5 Qualitatively, what is the effect of adding NaOH to an aqueous solution of NH₃as shown in the equilibrium reaction above?
NH ₄+ + OH - K b (NH ₃) = 1.8 10 - 5 Qualitatively, what is the effect of adding NaOH to an aqueous solution of NH₃as shown in the equilibrium reaction above?
(Use Le Chatelier s principle.)
Definitions:
Variable Input
An input whose quantity the firm can vary at any time to increase or decrease production.
Total Product Curve
The total product curve illustrates the relationship between the quantity of inputs used in production and the quantity of output produced, demonstrating the law of diminishing returns.
Variable Input
A production input whose quantity can be changed in the short term to adjust the level of output.
Marginal Product Curve
The marginal product curve depicts the change in output resulting from employing one more unit of a specific input, holding all other inputs constant, and typically features phases of increasing, constant, and diminishing marginal returns.
Q4: What is the pH of a solution
Q22: Consider the following reaction at equilibrium:<br>2 Cl₂
Q39: Consider the titration of a weak acid
Q39: What is the hydroxide ion concentration in
Q46: Consider the following reaction and its corresponding
Q49: What is the pH of 2.0 liters
Q56: For the reaction 2 KClO<sub>3</sub>(s) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8714/.jpg"
Q65: For a given chemical reaction, the value
Q78: If D H is positive and D
Q79: What is the IUPAC name for the