Using the entropy values in the table below, what is the change in standard entropy, D S , for the following reaction?
2 CH₃ OH (g) + 3 O₂(g) 2 CO₂ (g) + 4 H₂ O (g) Substance S (J/mol K) CH₃ OH ( 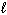 ) 126.8 CH₃ OH (g) 237.6 O₂(g) 205.0 CO₂ (g) 213.6 H₂ O (g) 188.83 H₂ O (
) 126.8 CH₃ OH (g) 237.6 O₂(g) 205.0 CO₂ (g) 213.6 H₂ O (g) 188.83 H₂ O ( 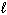 ) 69.91
) 69.91
Definitions:
Government Intervention
Actions by a government to affect the economy, often aiming to correct market failures or promote social welfare.
Imperfect Information
A market condition where all parties do not have equal access to all relevant information.
Life Insurance
A contractual agreement with an insurance company that provides a lump sum payment, known as a death benefit, to beneficiaries upon the insured person's death, in exchange for premium payments.
Market Failure
A scenario in which the distribution of goods and services through an unregulated market leads to inefficiencies, resulting in a decrease in overall social welfare.
Q7: The mass of an <sup>1</sup>H atom is
Q43: The oxidation state of S in SO<sub>2</sub>
Q55: What is the conjugate acid of the
Q75: Which of the following processes results in
Q93: Which of the following sulfates is least
Q103: What is the solubility of AgCl (s)
Q112: What is the value of the equilibrium
Q174: Employing a neutralization reaction , what reactants
Q186: Consider mixing 5.1×10<sup> - 6</sup> moles of
Q193: The reaction of chlorine and water vapor