Consider a mixture of three gases in the following reaction and the reaction s corresponding Standard Molar Gibbs Free Energy:
2 HI (g) 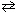 H₂ (g) + I2 (g) D G = 16.8 KJ/mol If the partial pressures of the three gases are P HI = 2.50 atm, PH 2= 3.5 atm and P I2 = 4.5 atm at 400 K, what is D G under these non-standard conditions ?
H₂ (g) + I2 (g) D G = 16.8 KJ/mol If the partial pressures of the three gases are P HI = 2.50 atm, PH 2= 3.5 atm and P I2 = 4.5 atm at 400 K, what is D G under these non-standard conditions ?
Definitions:
Debit
A bookkeeping entry that triggers an increment in assets or a decrement in liabilities on a corporation's financial statement.
Accounts Receivable
Funds that are due to a company from its customers for goods or services delivered on credit.
Fees Earned
Revenue earned from providing services to clients or customers, typically recorded in the accounting period when the services are rendered.
Invoice
A document issued by a seller to a buyer that details a transaction and requests payment for goods or services provided.
Q18: What is the pH of a buffer
Q40: The third law of thermodynamics states that:<br>A)
Q44: Refer to Exhibit 22-1. What is the
Q63: In the electrorefining process the impure metal
Q70: Which cyclic compound listed below is Cycloheptane
Q134: Pick out the ketone functional group.<br>A) <img
Q142: Refer to Exhibit 14-5. What would be
Q146: The conjugate acid of HPO<sub>4</sub><sup>2 - </sup>
Q156: From the list below, which compound is
Q185: Calculate the solubility of AgI in 0.10