Estimate the normal boiling point of mercury, given that for Hg ( 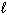 ) ,
) , 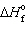 = 0 kJ/mol and D S = 76.02 J/mol K and for Hg (g) ,
= 0 kJ/mol and D S = 76.02 J/mol K and for Hg (g) , 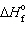 = 61.32 kJ/mol and D S = 174.85 J/mol K. The change is:
= 61.32 kJ/mol and D S = 174.85 J/mol K. The change is:
Hg ( 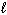 )
)  Hg (g)
Hg (g)
Definitions:
Schedule M-1
A form used by corporations filing U.S. federal corporate income taxes to reconcile financial statement income with tax return income.
Total Receipts
The total amount of money received by a business during a specific period, including sales, income, donations, and other forms of revenue.
Subchapter S Corporation
A type of corporation that meets specific Internal Revenue Code requirements and passes income, losses, deductions, and credits to its shareholders for federal tax purposes.
C Corporation
A legal structure for a corporation in which the owners, or shareholders, are taxed separately from the entity, leading to potential double taxation on earnings.
Q3: What is the pH of a solution
Q6: Under standard conditions the voltaic cell with
Q9: After balancing the following reaction under acidic
Q15: Which reaction shown below is shifted in
Q27: Which of the following titrations would produce
Q37: Which of the following compounds is considered
Q41: Which of the statements are true of
Q69: A sample of 100 mL of 0.10
Q87: Refer to Exhibit 15-3. What is the
Q113: Which of the following salts is(are) considered