Given the abbreviated table for selected standard molar entropy values of each substance in the reaction below, what is the standard molar change in entropy, D S rxn , for the following reaction?
3 NO₂ (g) + H₂ O ( 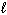 ) 2 HNO3(
) 2 HNO3( 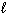 ) + NO (g) Substance S (Jmol - 1 K - 1 ) NO₂ (g) 239.9 H₂ O (
) + NO (g) Substance S (Jmol - 1 K - 1 ) NO₂ (g) 239.9 H₂ O ( 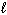 ) 69.94 HNO3(
) 69.94 HNO3( 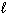 ) 155.6 NO (g) 210.65
) 155.6 NO (g) 210.65
Definitions:
Periodic Inventory System
An inventory accounting system where the inventory is physically counted at specific intervals to determine the cost of goods sold and ending inventory value.
Average Cost
An inventory costing method where all costs of inventory purchased are averaged to determine the cost of goods sold and ending inventory value.
Unit Cost
The cost incurred to produce, acquire, or deliver one unit of a product or service.
Q30: If only work of expansion is done,
Q47: Which of the following common aromatic compounds
Q50: Compare compounds I-III with compound A. Which
Q59: The reaction of ammonia and hydrogen chloride
Q64: The redox reaction (unbalanced), Cl<sub>2</sub>→ClO<sub>3</sub><sup> - </sup>
Q69: The molar solubility of SrF<sub>2</sub> in water
Q77: Start with the skeleton half-reaction CrO<sub>2</sub><sup> -
Q82: The only factor that causes a change
Q129: What is the pH of a 0.45
Q173: What can be stated about the name,