For the reaction 2 NO₂ (g) 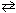 N₂O4 (g) , if a large amount of NO₂ (g) is injected into the reaction vessel containing NO₂ and N₂O4 at equilibrium, the instantaneous value of D G (before reaching the new equilibrium) is:
N₂O4 (g) , if a large amount of NO₂ (g) is injected into the reaction vessel containing NO₂ and N₂O4 at equilibrium, the instantaneous value of D G (before reaching the new equilibrium) is:
Definitions:
Desegregated Schools
Educational institutions that have been integrated, allowing students of all races to attend the same schools.
Montgomery Bus Boycott
A social protest campaign against racial segregation on the public transit system in Montgomery, Alabama, that began in 1955.
Women's Political Council
An organization instrumental in the Civil Rights Movement, particularly known for its role in organizing the Montgomery Bus Boycott in the 1950s in the United States.
Dr. Martin Luther King, Jr.
A prominent leader in the American civil rights movement, best known for his role in advancing civil rights using nonviolent civil disobedience based on his Christian beliefs.
Q25: At 25 ° C, K <sub>p</sub> =
Q30: Given the three statements below, pick the
Q35: Refer to Exhibit 17-3. What is the
Q48: Barium - 122 has a half-life of
Q66: In the titration of 0.100 M HCl
Q85: In the titration of 25.0 mL of
Q91: At 500 C the equilibrium concentrations are
Q119: Two anions act as bases. Anion A<sup>
Q122: Refer to Exhibit 15-3. What is the
Q139: Compare compounds I-III with compound A. Which