The equilibrium constant for the reaction N₂O4 (g) 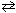 2 NO₂ (g) is 0.100 at 25 C. Calculate D G for this reaction. (R = 8.314 J/mol K)
2 NO₂ (g) is 0.100 at 25 C. Calculate D G for this reaction. (R = 8.314 J/mol K)
Definitions:
Singularity
Singularity refers to a hypothetical future point in time at which technological growth becomes uncontrollable and irreversible, resulting in unforeseeable changes to human civilization.
Strategic Decisions
High-level choices made by an organization's leadership that set the direction and scope of the company over the long term.
Marketing Strategy
A comprehensive plan formulated to achieve the marketing objectives of an organization, which includes target market identification and applying the marketing mix effectively.
Q36: What type of nuclear reaction is observed
Q47: The maximum number of unpaired electrons possible
Q49: Given the abbreviated table for selected standard
Q51: A water soluble salt composed of the
Q68: Given K p = 0.100 at 25
Q74: The mass defect of <sup>31</sup>P is 0.2822
Q78: A weak monoprotic acid is dissolved in
Q82: The pH at the equivalence point in
Q123: Which molecule from the list below is
Q153: The solubility of BaSO<sub>4</sub> is 1.14×10<sup> -