Consider the following reaction:
2 HCl (g) 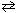 H₂ (g) + Cl₂ (g) Given the following table for the standard Gibbs Free Energy of formation for each substance in the previous reaction, what is the equilibrium constant, K , for this reaction at 298 K?
H₂ (g) + Cl₂ (g) Given the following table for the standard Gibbs Free Energy of formation for each substance in the previous reaction, what is the equilibrium constant, K , for this reaction at 298 K?
Substance D G f (kJmol - 1 ) HCl (g) - 95.30 H₂ (g) 0 Cl₂ (g) 0
Definitions:
Disaggregated Financial Data
Refers to financial information that is broken down into smaller components to provide detailed insight into the financial condition and performance of a company.
Risk of Investing
The potential for an investor to experience losses due to factors affecting overall financial market performance or specific asset classes.
Major Customers
Refers to clients who contribute significantly to a company's revenue, often making up a large portion of sales.
Auditor Opinions
Formal statements made by an auditor based on an audit of a company's financial statements, indicating the level of reliability of the financial information.
Q4: Starting from five carbons in a row
Q11: What is the solubility of Pb(IO<sub>3</sub>)<sub>2</sub> in
Q48: Consider the changes in acid strengths in
Q56: Which of these metals is not a
Q58: Refer to Exhibit 18-1. If electrons are
Q66: The combustion of a 0.440 g sample
Q107: What is the oxidation number for the
Q135: Consider the following molecules. Which of these
Q138: What common branching alkyl group is shown
Q146: The conjugate acid of HPO<sub>4</sub><sup>2 - </sup>