What is the value of the equilibrium constant, K , at 298 K for the following reaction?
Sn (s) + Pb 2+ (aq) 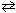 Sn 2+ (aq) + Pb (s) E cell = 0.01 V
Sn 2+ (aq) + Pb (s) E cell = 0.01 V
Definitions:
Net Operating Income
The profit a company makes after deducting operating expenses from gross profit, not including income and expenses from investments and interest.
Variable Costing
An accounting method that only considers variable costs in determining the cost of products.
Variable Costing
An accounting method that only includes variable production costs (materials, labor, and overhead) in product costs, excluding fixed costs.
Unit Product Cost
The total cost to produce one unit of product, including direct materials, direct labor, and a portion of all overhead costs.
Q18: What is the pH of a buffer
Q29: According to Egyptian theology, the pharaoh derived
Q44: What species are formed in the hydrolysis
Q58: Refer to Exhibit 15-1. What is the
Q76: Name the compound:<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8714/.jpg" alt="Name the compound:
Q89: The reaction below can be best described
Q96: What is the pH of an aqueous
Q96: A 30.0 mL sample of solution containing
Q100: Refer to Exhibit 17-2. Which statement below
Q102: Which of the following alkenes can have