What is the value of the equilibrium constant, K , at 298 K for the following reaction?
Sn (s) + Pb 2+ (aq) 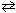 Sn 2+ (aq) + Pb (s) E cell = 0.01 V
Sn 2+ (aq) + Pb (s) E cell = 0.01 V
Definitions:
Self-Portraits
Artistic representations where artists create images of themselves, often used as a means of self-exploration or expression.
Rembrandt
A Dutch painter and etcher, one of the greatest artists in history, known for his masterful use of light and shadow.
Use of Light
The artistic and practical manipulation of light and shadows to enhance the visual experience of spaces or to achieve specific effects in photography, cinema, and art.
Netherlandish Painter
An artist from the regions corresponding to modern Belgium and the Netherlands in the 15th and 16th centuries, known for detailed naturalism in painting.
Q12: The alpha decay of <sup>231</sup>Pa produces:<br>A) "<sup>227</sup>Ac."<br>B)
Q13: Which of the following cannot behave as
Q15: Refer to Exhibit 18-3. Find the standard
Q27: Which of the following titrations would produce
Q41: Polonium - 218, a decay product of
Q47: Which process below is accompanied by a
Q47: The nuclide 226 Ra undergoes a series
Q55: A solution of HCOOH ( K <sub>a</sub>
Q55: In which of the complexes are optical
Q73: The combustion of a 3.06 g sample