A chemist was performing a spectroscopy experiment on a fluorescent dye to examine the influence of concentration on absorbance. To accomplish this, the chemist created a stock solution of a certain concentration of dye using 10 mL of water (H2O) . Then, a serial dilution was performed by taking 1 mL of the stock solution and diluting it with 9 mL of water for a total solution volume of 10 mL. 1 mL was taken from Solution 2 and diluted with 9 mL water to create Solution 3. The serial dilution was performed through Solution 5, as shown in the diagram below. If the dye concentration in Solution 5 was 0.5 g/mL, what was the concentration of the dye in Solution 2?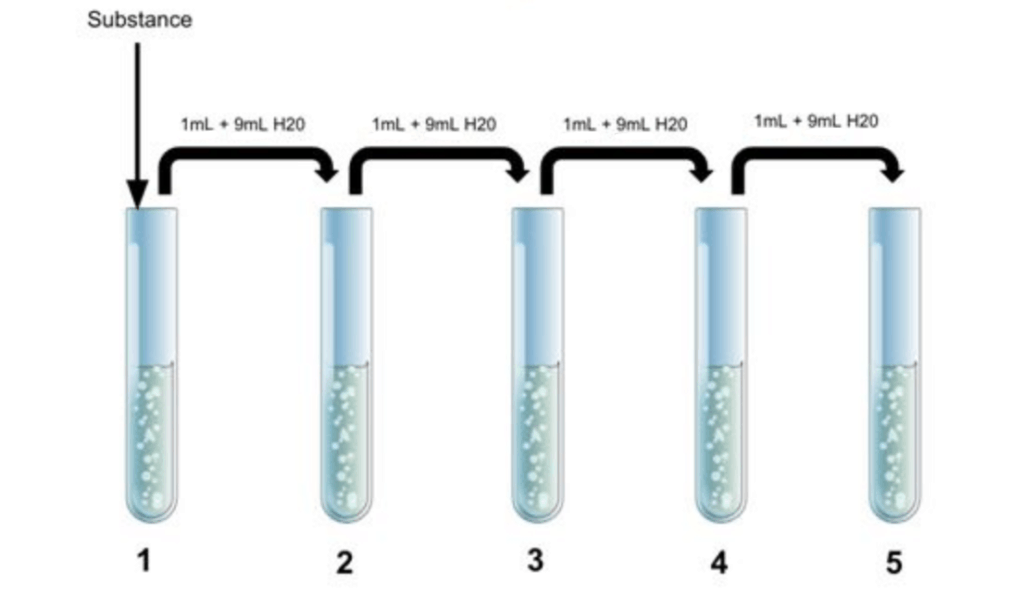
Definitions:
Halogens
A group of highly reactive elements located in Group 17 of the periodic table, which include fluorine, chlorine, bromine, iodine, and astatine.
Atomic Symbol
A one- or two-letter notation used to represent an element, based on its Latin name.
Copper
A red-orange metallic element that is ductile and conductive, used extensively in electrical wiring and plumbing.
Atomic Symbol
A one or two-letter notation representing a chemical element (e.g., H for hydrogen, O for oxygen) based on its name, often derived from Latin.
Q18: What is the other name for the
Q51: A boy is 11 years old and
Q59: Which of the following contains information on
Q71: Rachel Carson<br>Rachel Carson (1907-1964) was an American
Q77: Fine words butter no parsnips. Actions speak
Q109: DETER is the opposite of<br>A)Discourage<br>B)Encourage<br>C)Hinder<br>D)Dissuade<br>E)Prevent
Q155: BIRTH is to START as _?_ is
Q210: He who hesitates is lost. Fools rush
Q277: A friend call and tells you, "I
Q306: Which of the following is true about