The solution to the equation 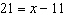 is
is
Definitions:
Isotopes
Variants of a particular chemical element which have the same number of protons in their nuclei but differ in the number of neutrons, leading to different atomic masses.
Atomic Number
The number of protons in the nucleus of an atom, which defines the chemical element.
Mass Numbers
The total number of protons and neutrons in the nucleus of an atom, indicating its atomic mass.
Subatomic Particles
Particles smaller than atoms, including protons, neutrons, and electrons, which are the building blocks of atoms.
Q5: Find the value of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8673/.jpg" alt="Find
Q8: Find the equation of a line with
Q129: Simplify the equation <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8673/.jpg" alt="Simplify the
Q154: Simplify the expression using the order of
Q192: The solution to the equation <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8673/.jpg"
Q239: The cash flow (in millions) of a
Q298: The half-life of Iodine-135 is 8 days.
Q302: Using the following table of a function
Q323: A 45-year-old woman comes to the office
Q346: Translate the phrase into an algebraic expression.<br>35