If we know the amount of light that is absorbed in a solution we can calculate the concentration of the substance dissolved in water using a spectrophotometer. For a certain substance the concentration (in moles/liter) is given by the formula  where
where 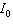 is the intensity of the incident light and
is the intensity of the incident light and 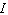 is the intensity of light that emerges. What is the concentration of the substance if the intensity
is the intensity of light that emerges. What is the concentration of the substance if the intensity 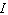 is 45% of
is 45% of 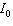 ?
?
Definitions:
Femininity
A collection of characteristics, actions, and functions typically linked to females by societal norms.
Smoking Preferences
Individual choices or tendencies related to the consumption of tobacco products, such as the type of product used, frequency, and context of smoking.
Peer Pressure
The influence exerted by a peer group, encouraging individuals to change their attitudes, values, or behaviors to conform to group norms.
Teaching Children
The process of imparting knowledge, skills, and social norms to young individuals to facilitate their growth and development.
Q18: Find the slope of the line given
Q23: A car dealer advertises a 35% discount
Q37: Given the solution set <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8673/.jpg" alt="Given
Q53: Using the definition of the logarithmic function
Q90: Using the graph below determine <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8673/.jpg"
Q107: Determine whether the equation <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8673/.jpg" alt="Determine
Q118: Determine the equation of the line that
Q125: Find the slope of the line that
Q163: Solve the inequality. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8673/.jpg" alt="Solve the
Q214: The equation <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8673/.jpg" alt="The equation