The following reaction represents the self-ionization of ammonia (NH3).
NH3(aq)+ NH3(aq) 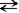 NH2-(aq)+ NH4+(aq)
NH2-(aq)+ NH4+(aq)
amide ammonium
Fill in the blanks with the appropriate terms from those listed below.
ammonia
ammonium
amide
-In this reaction, the conjugate base of ammonia is the _______________________ ion.
Definitions:
Word Root
The base part of a word, to which affixes (prefixes and suffixes) can be added to create related words.
Kidney
A pair of bean-shaped organs in the renal system that filter waste products from the blood and excrete them as urine.
Urinary System
The organs of the body involved in the production, storage, and excretion of urine.
Urine
A liquid waste product filtered from the blood by the kidneys, stored in the bladder, and expelled from the body through urination.
Q1: Which of the following redox coenzymes is
Q4: The prefix centi- denotes what fraction of
Q4: The correct structure for 3-hexanol is shown
Q40: Structure _ would from a thioester bond
Q48: The line structure for <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8894/.jpg" alt="The
Q59: In the formation of a thioester bond,
Q59: Oxalic acid (H<sub>2</sub>C<sub>2</sub>O<sub>4</sub>) is weak acid found
Q66: A pharmaceutical solution of penicillin contains 125
Q67: Which of the following reactions represents the
Q70: In which state of matter are the