Determine the end behavior of the function 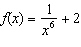 .
.
Definitions:
Hungarian Protest
Refers to various demonstrations or rebellions by the Hungarian people historically, often against government policies or foreign influence.
Discriminatory Policies
Practices or laws that unfairly discriminate against individuals or groups based on race, gender, age, nationality, religion, or other characteristics, leading to unequal treatment or opportunities.
Soviet Control
The political, military, and economic domination by the Soviet Union over various countries, particularly in Eastern Europe, during the Cold War.
Suez Crisis
The Suez Crisis was a diplomatic and military confrontation in late 1956 involving Egypt, Israel, France, and the United Kingdom, centered around the control of the Suez Canal, a key waterway for international trade.
Q2: An uncontrolled extraneous variable is<br>A)a confound<br>B)internally valid<br>C)externally
Q10: Sizing a system to provide all of
Q10: Which of the following statements is true
Q27: If <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8728/.jpg" alt="If and
Q47: Solve the system of linear equations. <img
Q56: Find the x -intercept(s) of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8728/.jpg"
Q56: The value of an investment at time
Q67: A ball is thrown straight upward from
Q67: The graph of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8728/.jpg" alt="The graph
Q82: Using the following table of a function