Exhibit 17-1
The following question(s) pertain to the electrochemical system pictured below. Cell A contains an aluminum electrode and a 1 molar solution of Al(NO3) 3. Cell B contains an iron electrode and a 1 molar solution of Fe(NO3) 2. 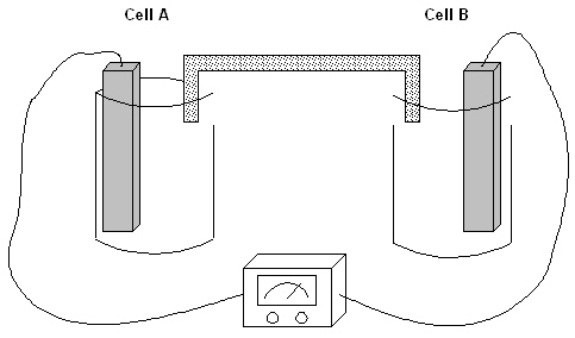
Refer to Exhibit 17-1. Which of the following is the chemical equation for the cell reaction that is spontaneous as written?
Definitions:
Q5: What +2 ion has the electronic configuration
Q10: Which of the follow are possible quantum
Q11: Exhibit 13-2<br>The following question(s) pertain to the
Q11: In the photoelectric effect, increasing the frequency
Q18: Exhibit 17-1<br>The following question(s) pertain to the
Q22: In general, DG, for dissolution reactions is
Q22: Which of these carbohydrates has the largest
Q25: The vibration frequency of HCl is 8.66×10<sup>13</sup>
Q26: A copolymer<br>A) is a polymer formed from
Q29: Exhibit 18-4<br>The following question(s) pertain to the