Multiple Choice
Which do you think has a higher boiling point, n-butanol or tert-butanol? 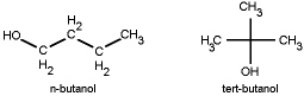
Definitions:
Related Questions
Q8: What is a benefit of conducting a
Q9: Name the following compound, Pt(H<sub>2</sub>NCH<sub>2</sub>CH<sub>2</sub>NH<sub>2</sub>)Cl<sub>4</sub>]Cl<sub>2</sub><br>A) hexachloro(ethylenediamine)platinum (IV)<br>B)
Q12: Exhibit 17-2<br>The following question(s) pertain to an
Q19: In DNA, genetic information is encoded by<br>A)
Q21: PVC for plumbing pipes can be made
Q22: A photoelectron spectrum of sodium excited with
Q23: For a body-centered unit cell, each corner
Q31: Oxidation of an aldehyde will result in
Q68: The trickster story "Red Willows" comes from
Q82: What site do some Christians associate with