Which do you think has a higher boiling point, n-butanol or tert-butanol? 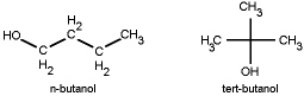
Definitions:
Free Market
An economic system where prices for goods and services are determined by the open market and consumers, with minimal government intervention.
Profit
The financial gain realized when the revenue gained from a business activity exceeds the expenses, costs, and taxes involved in sustaining the activity.
Green Initiative
Programs or actions undertaken to reduce environmental impact and promote sustainability.
Solar Panels
Devices that convert sunlight into electricity, consisting of photovoltaic cells that capture and transform solar energy.
Q2: An odds ratio can range from close
Q10: A student dissolves 30.00 grams of sodium
Q10: A coffee cup calorimeter is best used
Q12: The Born Openheimer approximation assumes that the
Q17: Distillation can be used to separate single
Q30: The conductivity of semiconductors can be increased
Q31: At 100 C, the following two
Q32: If the vibrational frequency of H<sup>35</sup>Cl is
Q36: At which famous site did virgin priestesses
Q42: What is the central issue of the