Refer to the figure shown.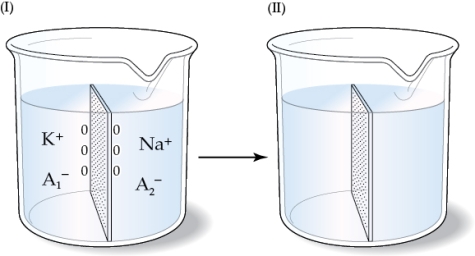 If the beaker on the left represents the initial state and the membrane shown in the beakers is permeable to K+ and Na+,
If the beaker on the left represents the initial state and the membrane shown in the beakers is permeable to K+ and Na+,
Definitions:
ΔH°
The standard enthalpy change of a reaction, indicating the overall heat absorbed or released under standard conditions.
Bond Dissociation Energies
The energy required to break a specific chemical bond in a molecule, indicating the strength of the bond.
Initiation Step
The first stage in a chemical reaction where reactants begin to form intermediates.
Free Radical Chlorination
A chemical reaction involving the substitution of hydrogen atoms in alkanes with chlorine atoms through the action of free radicals, typically used in organic synthesis.
Q2: Generic care is referred to as all
Q2: The three time frames over which animals
Q4: With more complicated patient populations worldwide, there
Q8: Why is phenotypic plasticity important in digestion
Q9: Assuming individuals of the same body size,
Q13: Animals, plants, and other objects in the
Q20: Refer to the figure shown.<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TBO1030/.jpg" alt="Refer
Q24: Antifreezes are synthesized primarily by<br>A) marine and
Q30: Which statement best describes blue whale feeding?<br>A)
Q45: When experimental animals are fed different diets