The following questions are based on the reaction A + B ↔ C + D shown in the figure below.
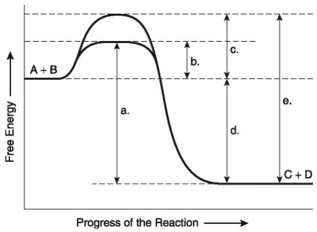
-Assume that the reaction in the figure above has a ΔG of -5.6 kcal/mol. Which of the following would be true?
Definitions:
Text Wrapping Setting
Configuration options in word processing and publishing software that determine how text flows around images, shapes, or other objects within a document.
Rotation Handle
The visual element in graphical interface applications that allows the user to rotate an object around a point.
Column Settings
Configuration options that determine the appearance, width, and other attributes of columns in documents, spreadsheets, or databases.
Newsletter
A regularly distributed publication, typically focused on a main topic, that is delivered electronically or by mail to subscribed members.
Q3: Which functional groups can act as acids?<br>A)amino
Q25: The structure labelled A is<br>A)very small.<br>B)hydrophilic.<br>C)hydrophobic.<br>D)NO.<br>E)a hormone.
Q26: The basis for diversity,both within a species
Q28: Compared to C₃ plants,C₄ plants<br>A)can continue to
Q34: If the carbon atom of the incoming
Q35: White blood cells engulf bacteria through what
Q62: The receptors for a group of signalling
Q71: Which of the following represents the difference
Q92: What is malonic acid's role with respect
Q100: Which of the following molecules contain(s)an aldehyde