Exhibit 6A This is a reaction going on in your muscle cells right this very minute: The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway. It follows simple Michaelis-Menten kinetics: Typical cellular concentrations: triose phosphate isomerase = 0.1 nM?dihydroxyacetone phosphate = 5 µM glyceraldehyde-3-phosphate = 2 µM

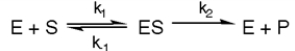
-Refer to Exhibit 6A. What is the equilibrium constant for the enzyme-catalyzed reaction?
Definitions:
Latent Content
In psychoanalytic theory, the underlying meaning of dreams as opposed to their manifest content, which is the dream as remembered by the dreamer.
Symbolic Content
Elements within dreams or other forms of communication that represent deeper, often unconscious, meanings or messages.
Lethal Overdose
The ingestion or exposure to a substance in quantities that exceed the level of tolerance in the body, leading to death.
Psychoactive Drugs
Substances that, when ingested, alter perception, mood, consciousness, cognition, or behavior by affecting brain function.
Q2: In allosteric interactions<br>A) proteins that consist of
Q11: Pyridoxal phosphate is required for transfer of<br>A)
Q12: The content of the NCLEX-PN examination is
Q19: Aspirin produces most of its analgesic effects
Q28: What is the charge on the tetrapeptide
Q29: Two dimensional separation methods<br>A) lead to unreliable
Q43: Plants need cholesterol in their membranes to
Q53: What provides the energy for rho-dependent chain
Q62: Transport of a compound across a
Q68: In the operation of the sodium-potassium pump<br>A)