Exhibit 6A This is a reaction going on in your muscle cells right this very minute: The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway. It follows simple Michaelis-Menten kinetics: Typical cellular concentrations: triose phosphate isomerase = 0.1 nM?dihydroxyacetone phosphate = 5 µM glyceraldehyde-3-phosphate = 2 µM

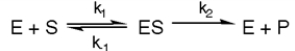
-Refer to Exhibit 6A. What is the equilibrium constant for the enzyme-catalyzed reaction?
Definitions:
Discouraged Workers
Refers to individuals who have stopped looking for work because they believe no jobs are available for them.
Unemployment Rate
The part of the labor force comprising people who are not employed and are actively seeking jobs.
Unemployment Rate
The measure of the percentage of the labor force that is unemployed and actively looking for employment.
GDP
Gross Domestic Product, the total value of all goods and services produced within a country over a specified period.
Q2: A student nurse is interested in working
Q9: The National League for Nursing (NLN) membership
Q12: Dressing for success at an interview means
Q13: When end-group analysis was done on the
Q24: The ion product constant for water (K<sub>w</sub>)
Q47: Topoisomerases are associated with<br>A) production of RNA
Q52: Refer to Exhibit 4B. The type of
Q59: Histones contain large amounts of which of
Q67: The degree of membrane fluidity depends on<br>A)
Q88: The association of membrane proteins with lipids