Figure 1.3
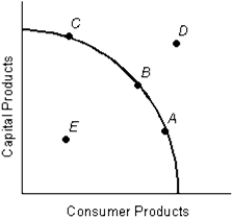
-When you work to support your lifestyle, you are making a tradeoff.
Definitions:
Reaction Rate
The speed at which reactants convert to products in a chemical reaction, influenced by factors like temperature and concentrations.
Reaction Rate
The speed at which a chemical reaction occurs, typically measured in terms of how fast a reactant is consumed or a product is formed.
Reactants Decrease
In a chemical reaction, it is the reduction in the concentration of reactant(s) as they are converted into product(s).
Products Increase
A term indicating that the amount of products formed in a chemical reaction increases over time.
Q15: A patient was identified as having cardiac
Q24: The following is an example of the
Q41: If the price elasticity of demand is
Q47: From a point of equilibrium, which of
Q61: If more consumers enter a market, the
Q66: According to the data in Table 2.3,
Q89: An increase in demand for healthy foods
Q98: For a free market to exist, economists
Q109: In the short run,<br>A) all the firm's
Q138: A boycott of lettuce would, if effective,