Use the table below to answer the following questions.
Table 11.2.3
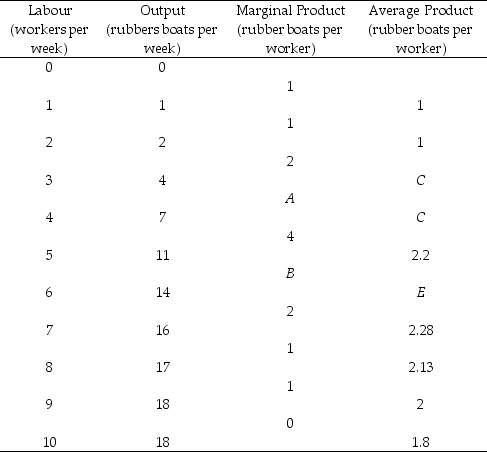
-Refer to Table 11.2.3. The value of A is
Definitions:
Homogeneous Catalyst
A catalyst that exists in the same phase (solid, liquid, or gas) as the reactants in a chemical reaction, promoting the reaction without being consumed.
Zero-order Reaction
A chemical reaction where the speed at which it occurs is unaffected by the amount of reactant(s) present.
Reactant Concentration
The amount of reactants present in a given volume of solution at the start of a chemical reaction, influencing the reaction rate.
First-order Reaction
A chemical reaction whose rate is directly proportional to the concentration of a single reactant.
Q17: Refer to Figure 1A.5.4. The graph shows
Q29: If firms in a perfectly competitive market
Q38: In the research and development game of
Q56: The output of a (not perfect)price-discriminating monopoly
Q65: A firm is producing the profit-maximizing amount
Q74: What kind of writing should a copywriter
Q82: A normative statement is a statement regarding<br>A)what
Q107: Refer to Table 12.2.3 which gives the
Q114: Good headlines interrupt readers' scanning and get
Q186: In Figure 1A.3.4, the slope across arc