Refer to the following figure to answer the following questions.
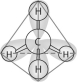
-In the methane molecule shown here, bonds have formed that include both the s orbital valence electrons of the hydrogen atoms and the p orbital valence electrons of the carbon. The electrons in these bonds are said to have
Definitions:
Working Capital
The disparity between a firm's immediate assets and liabilities, showcasing its short-term financial health and operational effectiveness.
Warehouse
A facility for storing goods that are being prepared for distribution or sale.
Profitability Index
A financial tool that calculates the ratio of the present value of future cash flows to the initial investment cost, determining the attractiveness of an investment.
Payback Period
The duration it takes for an investment to recoup its initial costs through profits or savings.
Q16: Atoms can be represented by simply listing
Q29: With rough sketches, draw a biological hierarchy
Q30: In an examination engagement for prospective financial
Q33: Which of the following takes place as
Q36: Which of the following is not a
Q45: Some bacteria are metabolically active in hot
Q61: Identical heat lamps are arranged to shine
Q80: What is the structural feature that allows
Q103: A CPA who has been engaged to
Q105: If an auditor discovers that previously issued