Draw Lewis structures for each hypothetical molecule shown below, using the correct number of valence electrons for each atom. Determine which molecule makes sense because each atom has a complete valence shell and each bond has the correct number of electrons. Explain what makes the other molecules nonsensical, considering the number of bonds each type of atom can make.
a.
c.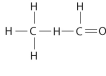
b.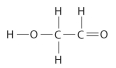
d. 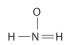
Definitions:
Union Bylaws
written rules and regulations governing the operation and management of a labor union, including membership requirements, officer duties, and meeting protocols.
Union Avoidance
Strategies and practices employed by employers to discourage workers from joining or forming unions.
U.S. Labor Law
The body of laws, administrative rulings, and precedents governing the legal rights of, and restrictions on, workers and their organizations in the United States.
Democratic Procedures
Processes and methods that follow the principles of democracy, typically involving inclusive participation, equal voting rights, and majority rule in decision-making.
Q8: Which type of bond must be broken
Q13: How many grams of the molecule in
Q25: Choose the term that correctly describes the
Q28: The majority of financial instruments are valued
Q30: The difference between pinocytosis and receptor-mediated endocytosis
Q51: How does a noncompetitive inhibitor decrease the
Q58: During glycolysis, when glucose is catabolized to
Q58: The standard letter of inquiry to the
Q62: Life is diverse. How many species are
Q84: Which of the following molecules could be