Refer to Figure 9.1 to answer the following questions.
Figure 9.1 illustrates some of the steps (reactions) of glycolysis in their proper sequence. Each step is lettered. Use these letters to answer the questions.
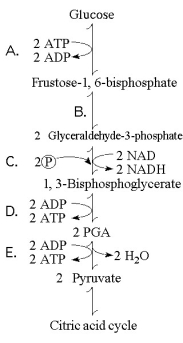
-The free energy for the oxidation of glucose to CO₂ and water is -686 kcal/mole and the free energy for the reduction of NAD+ to NADH is +53 kcal/mole. Why are only two molecules of NADH formed during glycolysis when it appears that as many as a dozen could be formed?
Definitions:
Program Evaluation
A thorough assessment of the effectiveness and efficiency of a program's objectives, design, implementation, and outcomes.
Review Technique
A systematic approach to assess, analyze, or evaluate a process, set of data, or document to ensure accuracy, completeness, or compliance with standards.
Coalitions
Alliances or unions between groups, typically for mutual benefit or to achieve a common goal.
Specific Problem
A clearly defined issue or challenge that requires a solution or resolution.
Q10: What is the mechanism for the production
Q35: Which structure is not part of the
Q36: Which of the following describes ubiquinone?<br>A)a protein
Q36: Tubulin is a dimer, made up of
Q37: One inhibitor of cGMP is Viagra. It
Q39: Thalidomide and L-dopa, shown below, are examples
Q44: Which of the following factors would tend
Q53: Testosterone functions inside a cell by<br>A)acting as
Q60: How many molecules of glucose (C₆H₂O₆ molecular
Q73: If a human interphase nucleus contains three