The following questions are based on the reaction A + B ↔ C + D shown in the figure below.
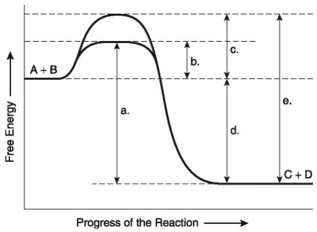
-Assume that the reaction in the figure above has a ΔG of -5.6 kcal/mol. Which of the following would be true?
Definitions:
Functional Structure
An organizational structure where the company is divided into departments based on their functions, such as marketing, operations, and finance.
Geographic Location
The specific physical position of something on the Earth's surface, determined by its latitude, longitude, and other factors.
Matrix Structure
An organizational structure that combines two or more types of organizational structures, most commonly functional and product-based divisions, aiming for greater flexibility in management.
Transnational Company
A multinational corporation that operates in multiple countries but adjusts its practices and products to local conditions.
Q23: Which of the events listed below occurs
Q39: The research team used the setup to
Q40: The drug cytochalasin B blocks the function
Q58: The cell walls of bacteria, fungi, and
Q59: The main purpose for the fermentation pathway
Q69: Which term most precisely describes the cellular
Q99: The basis for diversity, both within a
Q112: If cells are grown in a medium
Q115: Which of the following statements is true
Q117: Starting with one molecule of glucose, the